Can the gut really cause disease in the brain?
This web page was produced as an assignment for an undergraduate course at Davidson College.
Exposure to microbial proteins in the gastrointestinal tract accelerates the aggregation of insoluble proteins in the brain leading to debilitating diseases with severe neurological impairments and motor dysfunctions.
Amyloids. What are they? They are a unique protein class where aberrant accumulation leads to severe cellular dysfunctions associated with numerous neurodegenerative diseases. Synucleinopathies. What are they? They are a family of these amyloid diseases that include Parkison’s disease, Lewy Body disease, and Multiple System Atrophy, which are some of the most common neurodegenerative disorders. They are distinguished from other disorders by their protein abnormalities that correspond with abnormal conformational properties.^1 Central to the pathogenesis of these diseases is the accumulation of the neuronal protein alpha-Synuclein. This protein aggregates or accumulates into insoluble amyloid, which leads to severe inflammation and subsequent neuronal dysfunction. Over 20 years ago, this synaptic protein was identified as the primary component of the Lewy bodies that are a sine qua non of Parkinson’s disease (pictured below.) Since that time, extensive research has demonstrated that α-Synuclein pathology is not only a hallmark of Parkinson’s, but can also cause neuronal dysfunction and death.^2
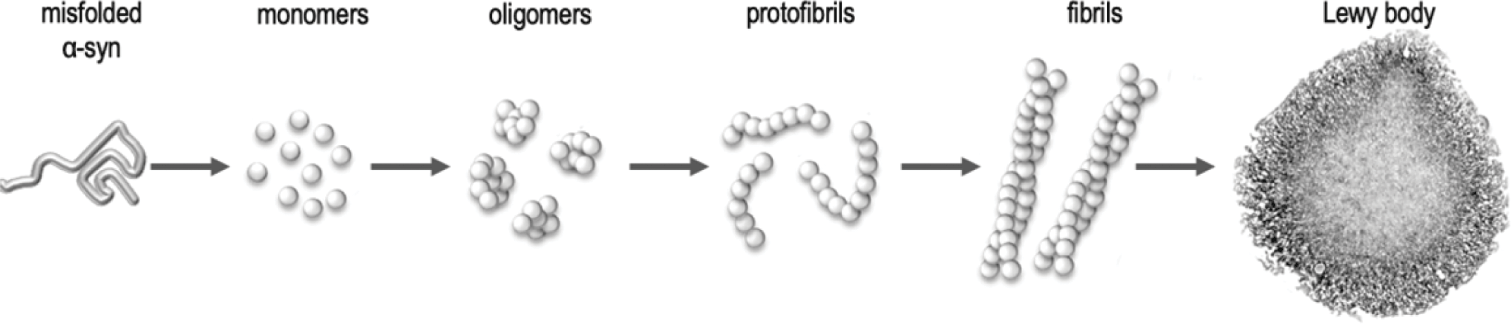
Accumulation of these aggregates first begins at peripheral sites in the gastrointestinal tract, before propagating via the vagus nerve or the spinal cord to sites in the brain. This makes logical sense because individuals diagnosed with various synucleinopathies often present with prior gastrointestinal dysfunctions years prior to the onset of movement difficulty. This connection has already been widely researched and studied among autism, depression, and Alzheimer’s disease. Idiopathic. What is that? It means being multi factorial and complex. Synucleinopathies are just that. Parkinson’s disease is just that. It is one of most disabling disorders of the central nervous system. The motor malfunctions of Parkinson’s include: shaking, rigidity, slowness of movement, postural instability and difficulty with walking and gait.^3 One such cause of this disease has been identified as these protein aggregates; however, it is important to keep in mind this is an idiopathic disorder and hence there are likely many other factors contributing to such a pathogenesis.
Back to the gut… What is going on? Or moreover what is going wrong? So the gut is colonized with a complex microbiome that impacts the function of many of the other human systems. A bacteria highly present within the human gut, enterobacteriaceae, produces functional amyloid proteins by the name of curli. These fibers are involved in adhesion to surfaces, cell aggregation, and biofilm formation. Curli fibers also mediate host cell adhesion and invasion.^4 Exposure of these fibers modulates the inflammatory response within the intestinal tract, especially in aged model species. Curli is abundantly expressed by certain gut bacteria, making it of significance in this paper. Researchers found that when mice were colonized to overexpress the alpha-Synuclein amyloid along with curli producing escherichia coli, the motor impairment and GI dysfunction of the model was exacerbated, providing a significant role for both components in aggregation. Behavioral deficits that resulted from these altered alpha-Synuclein pathways include both intestinal and motor impairments. This data was groundbreaking because it proves that the carriage of a particular bacterial taxa may exacerbate disease and raises provocative and meaningful future research questions.
A strength of this study was evidenced in how research was performed with several controls that compared the experimental group to a wild type. Data of the experimental group revealed note worthy exacerbated pathology and inflammation in comparison. A weakness in this study is that these results have been limited to a single transgenic mouse model and although providing hopeful results, the next step is to explore the incidences of curli encoding genes present in humans. All of the past, present, and future research in this area provides justification for future human studies and reveals new possible targets for intervention that may prevent, slow, or late amyloid formation and neurodegenerative disease. One such protein of high interest is tau. Mutations in tau implicate the initiation of neurodegeneration in deposits of the temporal and frontal lobes, regions that are very important for behavior and executive function.^5 Understanding this protein is critical because within the confines of this paper it responded differently from our primary protein of interest, alpha-Synuclein. Rather than production being accelerated in the disease, tau aggregation remained constant. This leads me to question what is different between these proteins? When and where is this protein malfunctioning? What is its pathology? Is it after the level of gene expression and protein formation?
Still with more questions, another area of interesting future study is evidenced in a plant derived dietary polyphenol by the name of Epigallocatechin gallate (EGCG) that physically inhibits amyloid formation. Assessment of motor performance revealed that EGCG improved both motor and GI defects exacerbated by curli producing e-coli. Not only does this plant have the capabilities to deter this aggregate formation, it has positive antioxidant and antinflammatory activities that prove beneficial for the health of the microbiome in and of itself, let alone implications for the health of the brain and neurological pathways later down the road. And finally, how can we further disentangle the complex relationships of how pathological signals from bacterial amyloids are transduced from the gut to the brain? Further exploring gut to brain signaling and the transneuronal spread and cascade of inflammatory aggregates will further provide means and mechanisms of further treatments and increased levels of protection against such debilitating disorders. This paper provides the spark for a potentially explosive fire in terms of future research, treatments, and cures.
References
- Dugger BN, Dickson DW. Pathology of Neurodegenerative Diseases. Cold Spring Harb Perspect Biol. 2017 Jul 5;9(7):a028035. doi: 10.1101/cshperspect.a028035. PMID: 28062563; PMCID: PMC5495060.
- Henderson MX, Trojanowski JQ, Lee VM. α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci Lett. 2019 Sep 14;709:134316. doi: 10.1016/j.neulet.2019.134316. Epub 2019 Jun 3. PMID: 31170426; PMCID: PMC7014913.
- Opara J, Małecki A, Małecka E, Socha T. Motor assessment in Parkinson’s disease. Ann Agric Environ Med. 2017 Sep 21;24(3):411-415. doi: 10.5604/12321966.1232774. Epub 2017 May 11. PMID: 28954481.
- Barnhart MM, Chapman MR. Curli biogenesis and function. Annu Rev Microbiol. 2006;60:131-47. doi: 10.1146/annurev.micro.60.080805.142106. PMID: 16704339; PMCID: PMC2838481.
- Pîrşcoveanu DFV, Pirici I, Tudorică V, Bălşeanu TA, Albu VC, Bondari S, Bumbea AM, Pîrşcoveanu M. Tau protein in neurodegenerative diseases – a review. Rom J Morphol Embryol. 2017;58(4):1141-1150.
Taylor Mingle is a senior at Davidson College expected to graduate with a Bachelor’s degree in Biology in May 2021. Contact her at tamingle@davidson.edu
© Copyright 2020 Department of Biology, Davidson College, Davidson, NC 28036