This web page was produced as an assignment for an undergraduate course at Davidson College
Intracellular NAD levels contribute to cancer cell proliferation and survival through gene amplification

Nicotinamide adenine dinucleotide (NAD) is a small molecule found in all living cells that is central to metabolism and maintaining cellular homeostasis. NAD functions as a cofactor—a substance that is essential to the activity of an enzyme—in redox reactions, supplying high energy electrons from one reaction to another. Other important roles of NAD include aging, the immune system, and DNA repair but when there is a dysregulation of NAD levels within cells, this may lead to metabolic diseases, aging-related diseases, defective immune responses, and cancer (Navas & Carnero, 2021). A major challenge to precision oncology has been developing drugs that effectively target the dysregulated metabolic landscape in cancer. The authors of this study aim to demonstrate that tissue context is the major determinant of dependence on the NAD metabolic pathway in cancer by analyzing thousands of tumor samples from different tissue types. The results suggest that the dependence on the NAD pathway in cancer arises from tissue-based gene amplification and enhancer remodeling. This is a crucial finding and a step towards developing personalized treatments that target NAD metabolism.
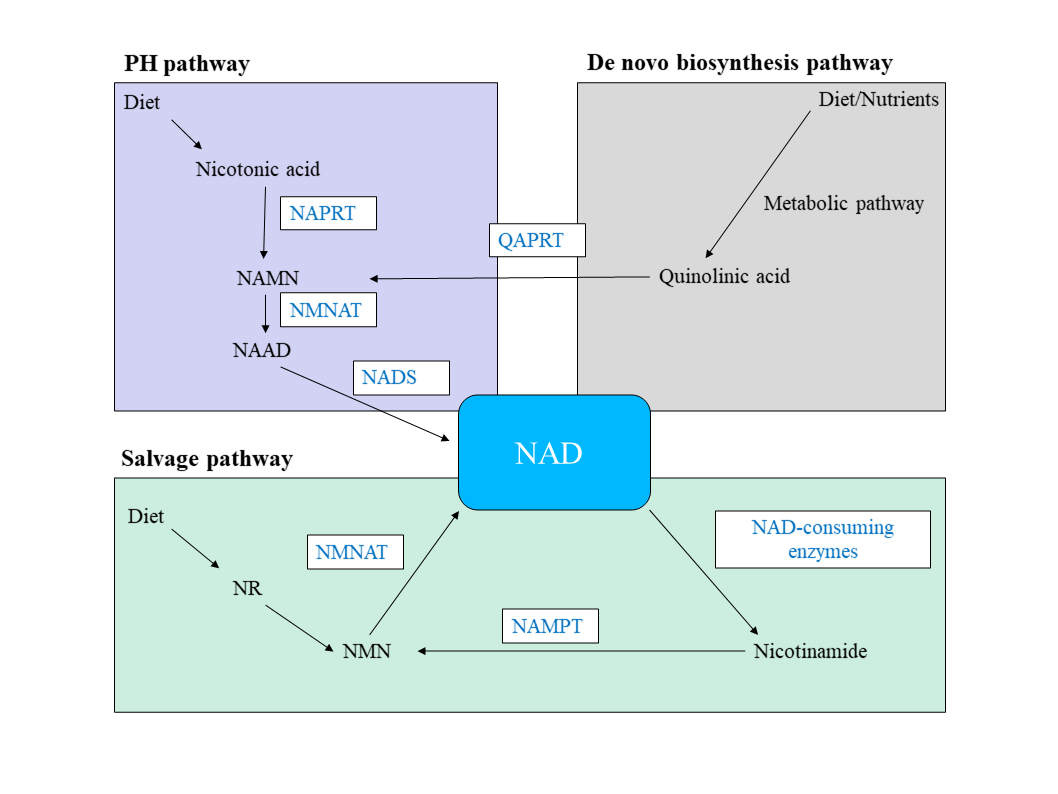
Organismal cells make NAD by three different, independent pathways: 1) de novo biosynthesis pathway; 2) salvage pathway, or 3) Preiss–Handler (PH) pathway (Figure 1). The salvage pathway is essential for maintaining cellular NAD levels and without this maintenance, cancerous cells are more likely to proliferate, survive, and metastasize (Navas & Carnero, 2021). Key enzymes of the PH and salvage synthesis pathway are NAPRT and NAMPT, respectively. These enzymes are rarely mutated, instead, their respective genes typically have an increase in DNA copy numbers in many cancers, thus leading to an increase in gene expression (Verdin, 2015). Consequently, NAD levels rise which permits the survival and quick proliferation of tumor cells since these cells depend on NAD for their glycolytic activity (Yaku et al., 2018).
The authors analyzed over 7,000 tumors of various tissue types and found that the amplification of a PH-pathway gene (NAPRT) was significantly correlated with NAPRT gene expression in 2,644 matched normal tissues from which those tumors arose (Chowdhry et al., 2019). Additionally, 93% of the NAPRT-amplified tumors arose from tissues that expressed high levels of the NAPRT transcript, indicating that the tissue context plays a role in determining which cancers amplify NAPRT. A key finding by the authors was that non-cancerous cells were able to depend on any of the NAD biosynthetic pathways to maintain cellular NAD levels, but cancerous cells did depend on a specific biosynthetic pathway. For instance, all the cancer cell lines with NAPRT amplification (PH-amplified) and none of the non-PH-amplified cell line depended on NAPRT for survival, suggesting that PH-amplified cancer cells are entirely dependent on the PH pathway for the maintenance of NAD and thus, cancer cell survival. In non-PH-amplified cancer cells, the biosynthetic pathway of choice appeared to be the salvage pathway in which cells were dependent upon NAMPT.
The results of ChIP-seq—a technique used to analyze protein interactions with DNA—revealed an enhancer for the NAMPT gene. This enhancer demonstrated potent activity in the salvage-dependent cancer cells, but not in the PH-amplified or non-cancerous cell lines, suggesting that the NAMPT enhancer drives NAD salvage-pathway selection in cancer cells. The discovery and analysis of this regulatory region was vital to demonstrating how NAD metabolic dependency is shaped in cancer because it showed that this enhancer controls the expression of NAMPT, regulates cellular NAD levels, and is required for tumor cell survival in salvage-dependent cancer cells. Together, it becomes clear that gene amplification and enhancer activity are driving forces for the success of cancer cells in tissues within specific biosynthetic NAD pathways. From this, inhibiting the expression of NAPRT will lead to tumoral levels of NAD plummeting and cancer cell death since NAPRT-amplified tumors use the PH-pathway for NAD maintenance survival. Similarly, the inhibition of NAMPT expression in non-PH-amplified tumors will lead to tumor regression and a decrease in NAD levels since these cells depend on the salvage pathway for the synthesis of NAD.
Future studies should focus on two main areas to deepen our understanding of cancer biology regarding the NAD metabolic landscape of cancer: 1) A comparative analysis of different enzyme inhibitors for NAPRT and NAMPT to determine which are the best suited for decreasing enzymatic activity and thus, decreasing NAD levels; 2) a closer analysis of the regulatory proteins that bind to the enhancer region of NAMPT to further elucidate how, or why non-PH amplified cancer cells depend on the NAD salvage pathway for survival. These questions will lead to significant progress towards precision oncology, however, a major barrier to clinical translation is deciphering a way to decrease NAD levels in the appropriate tissues and cells, while simultaneously not affecting healthy cells’ NAD levels since this homeostasis is essential for proper cellular function.
Dreylan Hines is an undergraduate student at Davidson College pursing a Bachelor’s degree in Biology. Contact him at drhines@davidson.edu.
© Copyright 2020 Department of Biology, Davidson College, Davidson, NC 28036.
Access the home page here.
References
Chowdhry, S., Zanca, C., Rajkumar, U. et al. NAD metabolic dependency in cancer is shaped by gene amplification and enhancer remodeling. Nature 569, 570–575 (2019). https://doi.org/10.1038/s41586-019-1150-2
Navas, L. E., & Carnero, A. (2021). NAD+ metabolism, stemness, the immune response, and cancer. Signal transduction and targeted therapy, 6(1), 2. https://doi.org/10.1038/s41392-020-00354-w
Verdin E. (2015). NAD⁺ in aging, metabolism, and neurodegeneration. Science (New York, N.Y.), 350(6265), 1208–1213. https://doi.org/10.1126/science.aac4854
Yaku, K., Okabe, K., Hikosaka, K., & Nakagawa, T. (2018). NAD Metabolism in Cancer Therapeutics. Frontiers in oncology, 8, 622. https://doi.org/10.3389/fonc.2018.00622