This web page was produced as an assignment for BIO309: Genomics at Davidson College.
SUrface-protein Glycan And RNA-seq, or SUGAR-seq, enables the detection and analysis of N-linked glycosylation, extracellular epitopes, and transcriptome at a single-cell level.
Glycosylation is a protein modification process when a carbohydrate chain is attached to a hydroxyl or a specific functional group (Kearney et al. 2021). For instance, during N-linked glycosylation, with the assistance of glycotransferases and glycosidases, glycans are sequentially added to the asparagine residues of the polypeptide. The resulted N-glycan chain would fold in specific manners, thus achieving the proper protein structure. Those final protein products play critical roles in cell development, signal transduction, cell migration, as well as immune homeostasis (Moremen et al. 2012). Given the significance of glycosylation, previous technologies, however, were incapable of capturing cellular glycosylation contents since they rarely focused on pose-translational properties (Buccitelli and Selbach 2020). To address such technical flaw, earlier this year, Kearny et al. developed a new technology called SUrface-protein Glycan And RNA-seq, or SUGAR-seq in abbreviation, which enables the detection and analysis of N-linked glycosylation as well as other phenotypic properties at a single cell level.
SUGAR-seq is based on the 10x feature barcoding technology platform, and it utilizes single-cell transcriptomics to read cellular properties. For target cells, surface proteins can be tagged with specific monoclonal antibodies conjugated to hashtag sequences. Glycoproteins, on the other hand, can be identified by different lectins, which are carbohydrate-binding proteins that selectively recognize sugar groups. In SUGAR-seq, biotinylated lectins are complexed with anti-biotin antibodies conjugated to oligonucleotides, so after cells are stained with those antibody complexes, glycans can be tagged with specific sequences. Therefore, similar to single cell RNA sequencing, protein and glycan abundance on cell surface are reflected through a sequencable readout of hashtags or oligonucleotides.
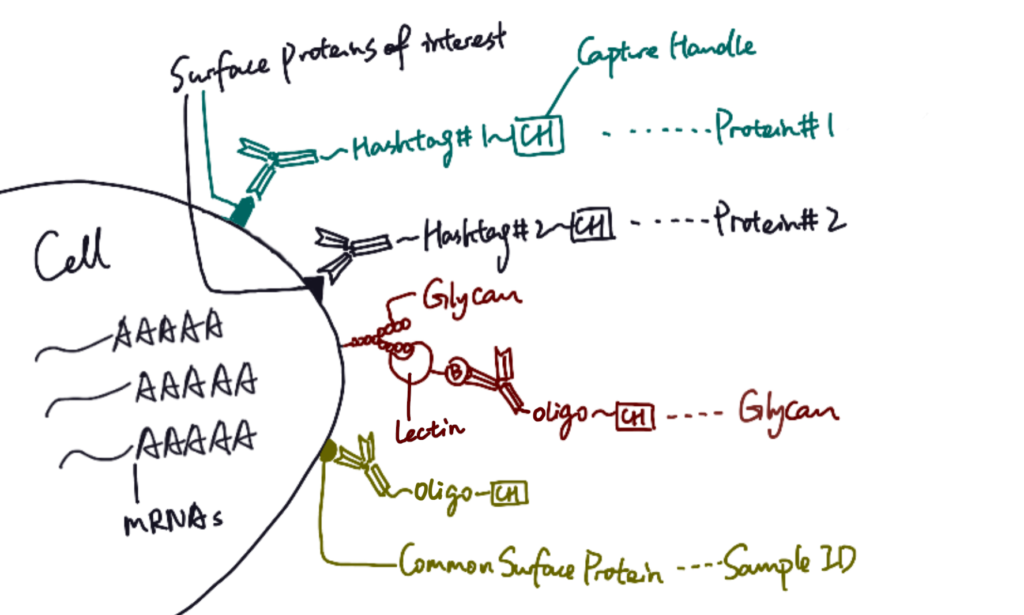
In their study, Kearney et al. applied SUGAR-seq to examine surface molecules of tumor-infiltrating T cells (TILs), which have been known to present high N-glycan contents (Marth and Grewal 2008). Specifically, two types of mice ovary tumor cells, B16-Ova and MC38-Ova, were selected for experiment (Kearney et al. 2021). Biotinylated Phaseolus vulgaris agglutinin (L-Pha), a type of lectin that specifically binds to complex-type N-glycan branches (Sharon and Lis 2004; Ghazarian et al. 2011), was chosen as the specific probe for the detection of glycan abundance on T cells. After cells were stained with antibody and lectin complexes, sequence tags on cell surfaces were analyzed with transcriptomics technology.
Uniform manifold approximation and projection (UMAP) was used to cluster transcriptomics results, which distinguished various T cell subpopulations according to identified hash-tagging. Analysis of L-Pha signal levels revealed divergent N-glycosylation levels among various TIL subsets: L-Pha signals were enriched in regulatory T cells (Treg) and exhausted T cells, showing that both were highly N-glycosylated, and on the other hand, memory T cells displayed low glycan abundance. To confirm the validity of SUGAR-seq results, researchers looked into combined outputs of various traditional technologies. FACS-based and FITC assays revealed glycosylation statuses that matched SUGAR-seq observations. Therefore, it was demonstrated that SUGAR-seq successfully distinguished T cell subsets with unique N-glycan profiles at the single-cell level.
SUGAR-seq can easily categorize cells into L-Phahigh or L-Phalow groups to reflect their N-glycan properties. In addition to the determination of glycosylation contents, SUGAR-seq, if combined to other genomic technologies, have expanded applications. As shown by Kearney et al., when applied together with proteomic analysis and Gene Ontology term analysis, SUGAR-seq could read proximity labeling and identify key T cell regulatory elements. For TILs, it discovered an enrichment of proteins involved in cellular adhesion and leukocyte activation in the L-Pha group. Moreover, with SUGAR-seq, glycosylation properties of T cell subgroups may assist the identification of distinct progresses in T cell cycle, or even lead to efficient disease diagnosis. According to previous immunology studies, during acute infections or vaccinations, naïve T cells will be activated and differentiate into effector T cells, some of which can then transition into memory T cells after the clearance of antigen. Alternatively, during chronic infections and cancer, under persistent antigen exposure, T cells differentiate into exhausted T cells (Wherry and Kurachi 2015). Analyzing the abundance of T cells in both L-Phahigh and L-Phalow groups, SUGAR-seq could tell the dominant T cell subsets and thus help us better understand the nature of immune responses observed. Here for TILs, as expected, exhausted T cells were highly enriched compared to another SUGAR-seq output of healthy lymph node cells, indicating tumor reactivity.
In conclusion, SUGAR-seq has been demonstrated to be an affordable, efficient, and comprehensive analysis technology to examine post-translational properties, including N-glycosylation, on cellular level. Further improvements may focus on expanding its applications to read products from other glycosylation pathways, or, since a single lectin is not able to detect all molecules of interest, the design of a reporter molecule panel. Nevertheless, SUGAR-seq has provided novel insights to the quantification of glycosylation products, and may help us investigate phenotypic or post-translational properties of cells in an efficient way. Its applications in disease diagnosis are appreciable, and we shall be looking forward to how SUGAR-seq will be integrated in more research studies in the future.
Author Info
Cindy Miao is a sophomore Chemistry Major and Data Science Minor at Davidson College. You may contact her through cimiao@davidson.edu.
Featured Image:
A specific type of lectin structure adapted from WikiMedia Commons.
References
Buccitelli C., and M. Selbach, 2020 mRNAs, proteins and the emerging principles of gene expression control. Nature Reviews Genetics 21: 630–644. https://doi.org/10.1038/s41576-020-0258-4
Ghazarian H., B. Idoni, and S. B. Oppenheimer, 2011 A glycobiology review: Carbohydrates, lectins and implications in cancer therapeutics. Acta Histochemica 113: 236–247. https://doi.org/10.1016/j.acthis.2010.02.004.
Kearney C. J., S. J. Vervoort, K. M. Ramsbottom, I. Todorovski, E. J. Lelliott, et al., 2021 SUGAR-seq enables simultaneous detection of glycans, epitopes, and the transcriptome in single cells. Science Advances 7: eabe3610. https://doi.org/10.1126/sciadv.abe3610.
Marth J. D., and P. K. Grewal, 2008 Mammalian glycosylation in immunity. Nature Reviews Immunology 8: 874–887. https://doi.org/10.1038/nri2417.
Moremen K. W., M. Tiemeyer, and A. V. Nairn, 2012 Vertebrate protein glycosylation: diversity, synthesis and function. Nature Reviews Molecular Cell Biology 13: 448–462. https://doi.org/10.1038/nrm3383.
Sharon N., and H. Lis, 2004 History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 14: 53R-62R. https://doi.org/10.1093/glycob/cwh122.
Wherry E. J., and M. Kurachi, 2015 Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15: 486–499. https://doi.org/10.1038/nri3862.