Comparing transcriptomics and proteomics data to discover distinct mechanisms of aging in the kidney
This web page was produced as an assignment for an undergraduate course at Davidson College.
Age-related changes at the protein and mRNA level provide evidence of aging processes that are not transcriptionally regulated.
The kidney is the body’s primary filtration system: this organ filters approximately half a cup of blood per minute, extracting waste and excess water to produce urine and flush this waste out (NIDDK 2018). Kidneys also act to balance levels of salt, sodium, potassium, calcium, and phosphorus in the blood, which is essential to maintain muscle and nerve function (NIDDK 2018). The kidney is composed of filtering units called nephrons, which have two main components: the glomerulus, which are tiny blood vessels that act as the filter, and the tubule, which removes the aforementioned excess fluid, waste, and acids (NIDDK 2018). Like many organs, kidneys decline with age, marked by decreased renal volume and a decline in glomerular filtration (Glassock et al. 2012). Kidneys are an ideal organ to study the aging process because its function is affected early in this process and it is non-invasive to measure protein and mRNA changes via simple blood and urine extractions (Lindeman et al. 1985). We know of physiological changes during aging, but little is known about underlying molecular processes (Takemon et al. 2021). Studies have explored these processes, but the study subjects were too specific or the experimental design had too many constraints to be applied as a generalized model for aging. We need a genetically diverse set of animals as well as an exploration of the association between transcriptional variation and changes in protein levels. Yuka Takemon et al. selected the kidney for further study of the difference in transcriptomic and proteomic data and assessed the extent in which these changes are associated in aging.
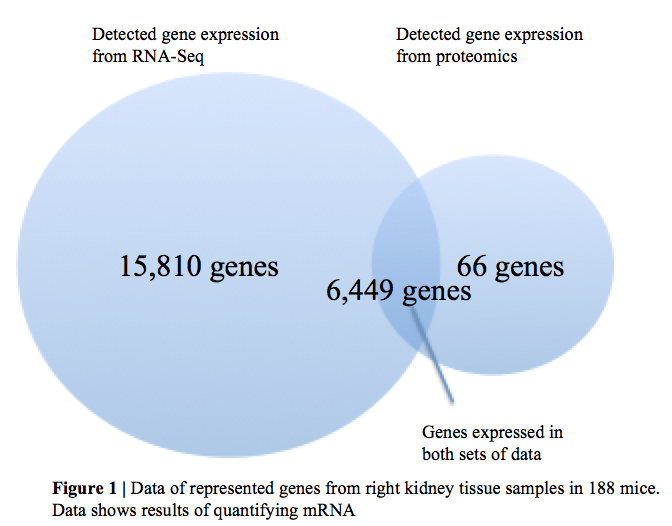
Takemon et al. measured mRNA and protein levels in genetically diverse mice at different age stages of 6, 12, and 18 months. They used 600 mice that were outbred from 8 founder strains that contribute over 50 million well-characterized variants (Takemon et al. 2021). Founder strains are used in genomics as reference strains and are of particular use because they account for a large amount of genetic diversity (Odet et al. 2015). This permitted researchers to observe common features of age-related change and provides a more well-distributed pool of data. Researchers collected urine from 490 mice and measured various minerals that are associated with kidney function to assess the organ’s function. Next they used flash-frozen tissue from 180 mice to quantify mRNA and proteins via RNA-Seq and untargeted proteomics data in order to see whether age or sex is the dominant factor in determining variation for these two molecular components. As seen in Figure 1, expression was detected for 22,259 genes from mRNA data, 6,515 genes from proteomics data, and data was available for both in 6,449 genes (Takemon et al. 2021). The 6,449 shared genes were used to generate principle component analysis plots, and these plots showed that sex was the dominant factor in determining mRNA variation, whereas age was the dominant factor in determining protein variation. The plots show that age is weakly correlated with mRNA variation, but that sex has a substantial correlation with protein variation, too. Variation of mRNA is constant across age groups, whereas age-specific variability of protein is greater in the oldest group of mice, indicating there is greater between-animal variation in protein at later ages (Takemon et al. 2021).
To get a closer glimpse of the age-related changes in mRNA expression, researchers utilized differential expression testing that only selects transcripts with the most notable age-related changes. This data revealed increased expression of the Cdkn2a gene among mRNA with the most significant age-related changes. This gene encodes two proteins, one of which is a hallmark for senescence (Hernandez et al. 2018). Senescence occurs when cell division terminates, but cells do not die. Other observations include an increased expression of genes that play a role in DNA damage repair and are involved in regulation of apoptosis, or cell death (Takemon et al. 2021).
In observing age-related changes in protein expression, researchers applied a linear model ANOVA to quantify protein abundance data. It is important to note, however, that protein abundance is not indicative of protein functional status, so we cannot make conclusions of whether these levels reflect proteins that are damaged vs. modified vs. functional, which is one limitation to this study (Takemon et al. 2021). One major finding showed an upregulation with age of transporters on the apical side of the kidney, and downregulation on the basolateral side of the tubule cell (Takemon et al. 2021). The basolateral side of the kidney relies on ion gradients produced by the sodium-potassium pump, whose cellular components are known to decrease with age, so this protein abundance data uncovered a very specific mechanism of aging in the kidney (Takemon et al. 2021). A second major finding revealed changes in actin remodeling with age. Actin acts to preserve the filtration properties of the glomerulus, so researchers suggest that actin remodeling may be indicative of changes in the filtration rate of the glomerulus as we age (Takemon et al. 2021).
Overall, the data demonstrates distinct differences in aging methods in the proteome and transcriptome. As we know, the RNA-seq data does not provide the whole picture: proteomics is required to take that data one step further to see what is actually making it to protein. We cannot deduce that changes in gene function, or protein levels, are observed from changes in mRNA (Takemon et al. 2021). This study provides clear evidence that the age-related changes in mRNA and protein expression differ, and that the type of age-related change of a protein cannot be predicted based on changes in the coding mRNA. Takemon et al. note that this applies even for genes that have an observed correlation between mRNA and protein within age groups (Takemon et al. 2021). A limitation of this study is that proteomics is catered to specific demands of specific cells. When considering the several different cell types that comprise the kidney, researchers were not able to conduct an experiment at a cell-type specific level (Takemon et al. 2021). A final limitation to consider is the age range of the study participants: 18 months is not very old when considering a mouse’s lifespan. One should consider including mice of older ages to better encompass the life aging process and observe how these mechanisms are impacted by age.
These findings suggest that changes with age at the protein and mRNA level are influenced by different factors, and understanding the mechanisms that separate gene expression and protein expression are crucial to improving our knowledge of what drives organs to be more susceptible to disease with age (Takemon et al. 2021). Protein-related changes are not influenced by changes in mRNA; rather, the researchers suggest that protein changes are often influenced by the changes in protein homeostasis that is associated with older animals (Takemon et al. 2021). As a result, this research could lead us to develop better targeted therapies to fight these diseases that are more likely to impact us as we age.
Svenja Nanfelt is a senior biology major at Davidson College. Contact her at svnanfelt@davidson.edu.
References
Your Kidneys & How They Work. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/kidney-disease/kidneys-how-they-work. (2018).
Takemon Y, Chick JM, Gerdes Gyuricza I, Skelly DA, Devuyst O, et al. Proteomic and transcriptomic profiling reveal different aspects of aging in the kidney. Elife. 2021 Mar 9;10:e62585. doi: 10.7554/eLife.62585. Epub ahead of print. PMID: 33687326.
Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of decline in renal function with age. J American Geriatric Society. 1985 Apr;33(4):278-85. doi: 10.1111/j.1532-5415.1985.tb07117.x. PMID: 3989190.
Glassock RJ, Rule AD. The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 2012 Aug;82(3):270-7. doi: 10.1038/ki.2012.65. Epub 2012 Mar 21. PMID: 22437416; PMCID: PMC3513938.
Odet, F. et al. The Founder Strains of the Collaborative Cross Express a Complex Combination of Advantageous and Deleterious Traits for Male Reproduction. G3 (Bethesda)5, 2671–2683 (2015).
Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018 Jun;28(6):436-453. doi: 10.1016/j.tcb.2018.02.001. Epub 2018 Feb 21. PMID: 29477613.
Return to home.
© Copyright 2021 Department of Biology, Davidson College, Davidson, NC 28036.